Abstract
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) is a life-saving procedures for many hematological diseases. However, its toxicity counterbalances its curative potential. Several prognostic scores have been created in the last two decades. Most scores derive from small sized and monocentric cohorts of patients. Also, contemporary patients and alternative donors are usually excluded from these scores.
Methods
We selected patients from the EBMT registry with the following characteristics: age > 18 years old; allogeneic hematopoietic cell transplantation (alloHCT) performed between 2010 and 2019; hematological disease diagnosis (AML/ALL; MDS/MPN; NHL/HL; plasma cell dyscrasias); any disease status at transplant; use of HLA-matched (familiar or unrelated) or haploidentical donor; myeloablative or RIC/NMA conditioning regimen; peripheral blood or bone marrow graft source. The primary objective was to create a prognostic score able to predict overall mortality (OM) at +2 years. The secondary objective was to predict non-relapse mortality (NRM) at 2 years. To create a newer prognostic score, we explored the following statistic and machine-learning techniques: step-wise logistic regression, support vector machine with linear and radial kernels, random forest, gradient boosting, elasticnet. We also tested simple fully connected neural network. The score included all the pre-transplant variables with a well-known prognostic role in terms of NRM related to disease (disease type) patient (age, gender, Karnofsky score, body mass index, presence of significant comorbidities, CMV status) transplant (time from diagnosis to transplant, donor type, GVHD prophylaxis, conditioning intensity, graft type) and transplant center characteristics (JACIE accreditation, > 20 alloHCT/year). OM model development was made on 70% of the data set, optimized on 15% and validated on the 15% of remaining data. Models with the best accuracy were selected to build the prognostic score.
Results
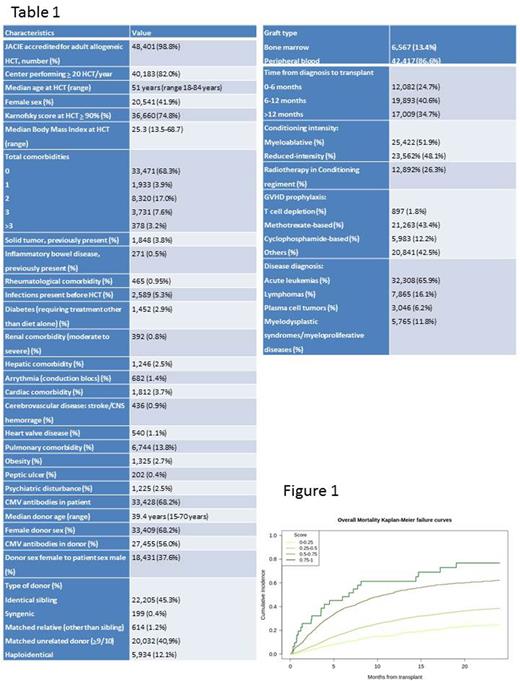
A total of 48984 patients were included into the analysis (Table 1). Median follow-up time for survivors was 38.4 months. Overall mortality and NRM at +2 years were 39.5% (95% CI=39.0-40.0%) and 19.0% (95% CI=18.5-19.5%), respectively. The 5 models predicted 2-year OM and NRM with similar AUC values. Best models were, for OM, gradient boosting (XGBoost) with AUC=0.64 (95% CI 0.630-0.655) and for NRM, elasticnet with AUC=0.58 (0.566-0.594). Neural networks had slightly higher performance for NRM but has limitations regarding the explicability of results. Same results were observed also when performing the analysis with the haploidentical cohort (n=5.934, AUC 0.66 for OM and 0.57 for NRM). In the final prognostic score including all the variables, gradient boosting was used to predict OM and elasticnet to predict NRM. The score was able to furnish a personalized prediction for each patient with continuous values. Patients with the lowest score had a 2-year OM and NRM of 22.2% and 8.7%, respectively. Patients with the highest score had a 2-year OM and NRM of 81.2% and 87.1%, respectively. A visual representation of the score prediction distribution was made on the validation cohort grouping the potential results into 4 groups (figure 1).
Conclusion
Our score showed to be useful in improving risk stratification of OM and NRM in the current era, including haploidentical transplants and post-transplant cyclophosphamide GVHD prophylaxis which were not included in previous scores. However, a generally poor accuracy despite the robust methodology confirms previous observations highlighting that the results of a prognostic score itself should not be used to exclude patients from a life-saving procedure. In such high-risk cases, more comprehensive evaluations (e.g. geriatric scores), biological scores (e.g. EASIX scores) and the overall patient's clinical phenotype should be evaluated to establish the actual transplantation risk and help in this difficult therapeutic choice
Disclosures
Mussetti:GILEAD: Research Funding; BMS: Consultancy; TAKEDA: Honoraria; JAZZ PHARMACEUTICALS: Consultancy. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Forcade:MSD: Other: Travel Support; Gilead: Other: Travel Support, Speakers Bureau; Jazz: Other: Travel Support, Speakers Bureau; Novartis: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Other: Travel Support. Platzbecker:Geron: Honoraria; BMS/Celgene: Honoraria; Janssen: Honoraria; Silence Therapeutics: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; Jazz: Honoraria; Novartis: Honoraria. Angelucci:Vifopr: Honoraria, Other: Data monitoring committee; Sanofi: Speakers Bureau; Vertex: Honoraria, Other: Data monitoring committee; Roche: Consultancy; Gilead: Consultancy; Glaxo: Consultancy; Bluebird Bio: Consultancy; Celgene: Honoraria, Other: Data monitoring committee; Novartis: Honoraria; Menarini/Stemline: Consultancy. Chevallier:Incyte: Research Funding; Takeda: Honoraria; Pfizer: Research Funding; Abbvie: Honoraria; Jazz Pharmaceuticals: Honoraria. Yakoub-Agha:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Janssen: Honoraria; Bristol Myers Squibb: Honoraria. Craddock:Novartis: Consultancy; Abbvie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy. Ciceri:Kite Pharma: Consultancy. Schroeder:Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Moiseev:Janssen: Honoraria; Jazz: Honoraria; Takeda: Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Penack:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Equillium Bio: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees; Priothera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Therakos: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees. Schoemans:Incyte, Janssen, Novartis , Jazz Pharmaceuticals, Takeda: Other: Personal Fees; Belgian Hematological Society (BHS): Other: Fees paid to institution; Novartis and the BHS (Belgian Hematological Society): Research Funding; serEBMT, EUPATI (the European Patient academy): Other: serves regularly as a volunteer for EBMT and occasionally for EUPATI (the European Patient academy). Mohty:Astellas: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria; Novartis: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria. Glass:Gilead: Consultancy; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Sureda:ROCHE: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; BMS: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; MSD: Honoraria; TAKEDA: Consultancy, Honoraria, Speakers Bureau; SANOFI: Consultancy, Honoraria; GILEAD: Consultancy. Basak:Gilead: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Saventic Health: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Human Biome Institute: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal